-
About Us
-
Business
-
IR/PR
-
Careers
-
CS

We will become the leader in the chemical field based
on our best technologies
The chemical division of KOLON Life Science consists of divisions including Active Pharmaceutical Ingredients, Specialty Chemical,
and Water Solution.

-
- Active Pharmaceutical Ingredients
- Provide APIs and intermediates for pharmaceutical companies worldwide from Kolon’s newest GMP compliant state-of-the-art facility
-
- Specialty Chemical
- Supply pyrithione antimicrobials used for dandruff shampoos and antifouling agents to global clients
-
- Water Solution
- Provide the total solution related to various water treatment chemicals based on polymer coagulants to sewage treatment plants and waste water disposal plants
The chemical R&D center will develop excellent products including generic APIs, intermediates for pharmaceuticals and polymer coagulants based on its experience in the field of synthesis technologies and convergence technologies
Our Key Technologies
-
 FT-NMR
FT-NMR -
 HPLC, HPLC-MS, GC
HPLC, HPLC-MS, GC -
 XRD, FT-IR
XRD, FT-IR -
 PSD
PSD -
 Dispersion stability analyzer
Dispersion stability analyzer -
 SEM
SEM
HPLC-MS : high-performance liquid chromatography - Mass Spectrometer
GC: Gas chromatography
-
 Incubator
Incubator -
 Fermentor
Fermentor -
 High pressure homogenizer
High pressure homogenizer -
 Laminar reactor
Laminar reactor -
 FBRM
FBRM
-
 FT-NMRFT-NMRFourier transform - Nuclear magnetic resonance
FT-NMRFT-NMRFourier transform - Nuclear magnetic resonance -
 HPLC, HPLC-MS, GCHPLC, HPLC-MS, GCHPLC : high-performance liquid chromatography
HPLC, HPLC-MS, GCHPLC, HPLC-MS, GCHPLC : high-performance liquid chromatography
HPLC-MS : high-performance liquid chromatography - Mass Spectrometer
GC: Gas chromatography -
 XRD, FT-IRXRD, FT-IRX-Ray diffraction
XRD, FT-IRXRD, FT-IRX-Ray diffraction -
 PSDPSDParticle size distribution analyzer
PSDPSDParticle size distribution analyzer -
 Dispersion stability analyzerDispersion stability analyzer
Dispersion stability analyzerDispersion stability analyzer -
 SEMSEMScanning Electron Microscope (SEM)
SEMSEMScanning Electron Microscope (SEM)
-
 IncubatorIncubator
IncubatorIncubator -
 FermentorFermentor
FermentorFermentor -
 High pressure homogenizerHigh pressure homogenizer
High pressure homogenizerHigh pressure homogenizer -
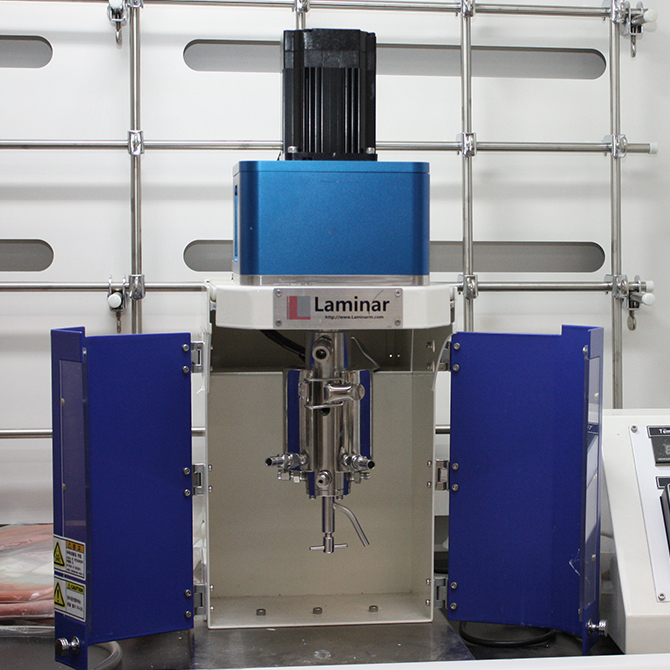 Laminar reactorLaminar reactor
Laminar reactorLaminar reactor -
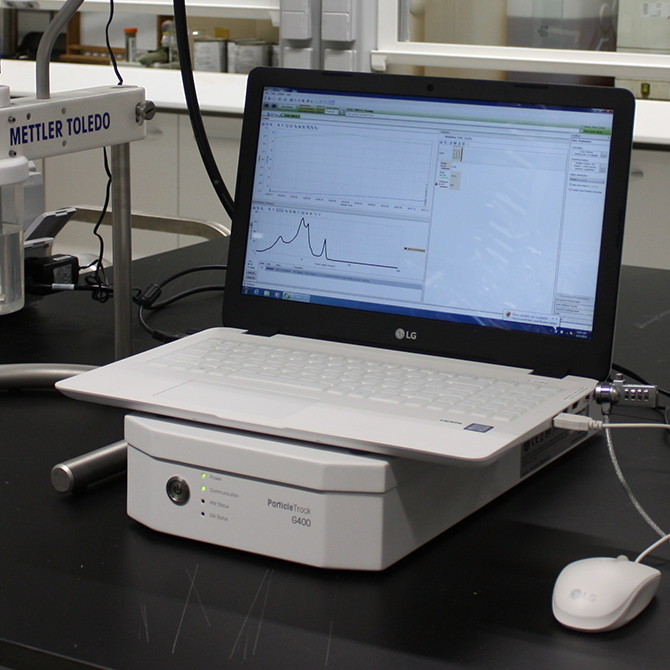 FBRMFBRMFocused Beam Reflectance Method
FBRMFBRMFocused Beam Reflectance Method
The API plant was completed in 2012 and is equipped with the newest facilities. It has been recognized as one of the leading API production plants in Asia. This plant is operated in two separate buildings to ensure stable supply.

- Products
- Various active pharmaceutical ingredients including anti-inflammatory analgesic drugs, antihypertensive drugs and anti-diabetics are produced through the processes including reaction, crystallization, filtration, dehydration, pulverization and packaging.

- Production capacity
- The plant is equipped with stainless steel and glass-line type reactors in various sizes including 300L and 10,000L. 750 tons of active pharmaceutical ingredients per year and 300 tons of intermediates for pharmaceuticals per year can be produced.

The GMP system is operated and the BGMP approval was received from MHLW (Japanese Ministry of Health, Labour, and Welfare) and MFDS in 2013.
| Type | Certifications | Certificate authority and country | Year and month of acquisition |
|---|---|---|---|
| ESH | ISO 45001:2018 | Societe Generale de Surveillance(SGS) | 2020.06 |
| OHSAS 18001:2007 | Soiete Generate de Surveillance(SGS) | 2017.11 | |
| ISO 14001:2015 | Soiete Generate de Surveillance(SGS) | 2017.12 | |
| API | Approval of Pharmaceutical Manufacturing License | MFDS / Korea | 2012.11 |
| Approval of Accreditation of foreign drug manufacturer | MHLW / Japan | 2013.3 | |
| Certificate of GMP Compliance of a Manufacturer (Synthesis) | MFDS / Korea | 2013.6 | |
| Certificate of GMP Compliance of a Manufacturer (Extraction) | MFDS / Korea | 2018.6 | |
| Inspection for GMP Compliance of a Manufacturer | PMDA / Japan | 2018.12 |
| API |
Certifications : OHSAS 18001:2007
Certificate authority and country : Soiete Generate de Surveillance(SGS)
Year and month of acquisition : 2017.11
Certifications : ISO 14001:2015
Certificate authority and country : Soiete Generate de Surveillance(SGS)
Year and month of acquisition : 2017.12
|
|---|---|
| Quality |
Certifications : Approval of Pharmaceutical Manufacturing License
Certificate authority and country : MFDS / Korea
Year and month of acquisition : 2012.12
Certifications : Approval of Accreditation of foreign drug manufacturer
Certificate authority and country : MHLW / Japan
Year and month of acquisition : 2013.3
Certifications : Certificate of GMP Compliance of a Manufacturer (Synthesis)
Certificate authority and country : MFDS / Korea+
Year and month of acquisition : 2013.6
Certifications : Certificate of GMP Compliance of a Manufacturer (Extraction)
Certificate authority and country : MFDS / Korea
Year and month of acquisition : 2018.6
Certifications : Inspection for GMP Compliance of a Manufacturer
Certificate authority and country : PMDA / Japan
Year and month of acquisition : 2018.12
|
The quality control tests for APIs and intermediates product are carried out here. 15 separate laboratories including an instrumental analysis laboratory, stability laboratory, microbiology laboratory and record center (document storage) are operated according to testing purposes.
The laboratories have approximately 40 physico-chemical and microbiological analysis instruments including HPLC, GC, UV and IR.
Approximately 20 high quality active pharmaceutical ingredients are produced based on experience and know-how accumulated over decades and the plant design is divided into two separate production buildings, thus supplying products to customers in a stable manner.
Various active pharmaceutical ingredients including anti-inflammatory analgesic drugs, antihypertensive drugs and anti-diabetics are produced through the processes including reaction, crystallization, filtration, dehydration, pulverization and packaging.
The GMP certification was received in 1998, and compliance with relevant standards and procedures was verified by Portugal Infarmed in 2010 and PMDA in 2011 based on site inspections.
| Type | Certification | Certificate authority and country | Year and month of acquisition |
|---|---|---|---|
| API | Certificate of GMP Compliance of a Manufacturer | MDFS / Korea | 1999.11 |
| Inspection for GMP Compliance of a Manufacturer | PMDA / Japan | 2011.4 | |
| Quality | Inspection for GMP Compliance of a Manufacturer | PMDA / Japan | 2015.7 |
| Inspection for GMP Compliance of a Manufacturer | PMDA / Japan | 2019.2 |
| API |
Certifications : Certificate of GMP Compliance of a Manufacturer
Certificate authority and country : MDFS / Korea
Year and month of acquisition : 1999.11
Certifications : Inspection for GMP Compliance of a Manufacturer
Certificate authority and country : PMDA / Japan
Year and month of acquisition : 2011.4
|
|---|---|
| Quality |
Certifications : Inspection for GMP Compliance of a Manufacturer
Certificate authority and country : PMDA / Japan
Year and month of acquisition : 2015.7
Certifications : Inspection for GMP Compliance of a Manufacturer
Certificate authority and country : PMDA / Japan
Year and month of acquisition : 2019.2
|
Gimcheon plant produces polymer coagulant used in the whole water treatment field including the treatment of service water, industrial water, industrial wastewater and sewage, along with a plant that produces pyrithione used as an antifoulant for lower hull paints, antimicrobial for plastics, and anti-dandruff agent for shampoo.

| Type | Certification | Certificate authority and country | Year and month of acquisition |
|---|---|---|---|
| ESH | ISO 14001:2015 | Soiete Generate de Surveillance(SGS) | 2017.12 |
| ISO 45001:2018 | Soiete Generate de Surveillance(SGS) | 2020.06 | |
| Quality | ISO 9001:2015 | Soiete Generate de Surveillance(SGS) | 2018.6 |
| EFfCI GMP for Cosmetic Ingredients(2017) | Soiete Generate de Surveillance(SGS) | 2018.6 |
| ESH |
Certifications : ISO 14001:2015
Certificate authority and country : Soiete Generate de Surveillance(SGS)
Year and month of acquisition : 2017.12
Certifications : ISO 45001:2018
Certificate authority and country : Soiete Generate de Surveillance(SGS)
Year and month of acquisition : 2020.06
|
|---|---|
| Quality |
Certifications : ISO 9001:2015
Certificate authority and country : Soiete Generate de Surveillance(SGS)
Year and month of acquisition : 2018.6
Certifications : EFfCI GMP for Cosmetic Ingredients(2017)
Certificate authority and country : Soiete Generate de Surveillance(SGS)
Year and month of acquisition : 2018.6
|
-
Gimcheon Plant 1Powder CoagulantProducts
This plant produces polymer flocculant in powder form.
Production capacityThe plant is operated 24 hours a day with processes including polymerization, chopping, drying, sieving and packaging taking place. The plant has a production capacity of 10,000 tons a year.
-
Gimcheon Plant 2
-
 Liquid CoagulantProducts
Liquid CoagulantProductsThis plant produces coagulant in liquid form.
Production capacityIt has a production capacity of 6,000 tons a year. flocculant is produced through processes including monomer make-up, emulsification, polymerization, inverting, filtering and packaging, and the product is packaged in 20kg cans, 1-ton IBC and 15-ton and 20-ton tank lorries.
-
 High-purity PyrithioneProducts
High-purity PyrithioneProductsThis manufacturing plant produces high-purity pyrithione used as antifoulant for lower hull paints and antimicrobial for plastic.
Production capacityHigh-purity pyrithione has been produced for 20 years through particle size and shape control technologies based on the study of morphology. Customer-specialized products in forms including powder, paste, solution and dispersion are provided to customers.
-
 Zinc Pyrithione SuspensionProducts
Zinc Pyrithione SuspensionProductsThis manufacturing plant produces zinc pyrithione suspension used as anti-dandruff agent for shampoo.
Production technologyThis plant is currently partnering with a global company through in relation to preservative formulation technology that overcomes the disadvantage of a single material and maximizes the antibacterial effect.
-